Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Mori, Michiyasu; Ziman, T.*
IEEE Transactions on Magnetics, 59(11), p.1300505_1 - 1300505_5, 2023/11
Times Cited Count:0 Percentile:0(Engineering, Electrical & Electronic) Zn
Zn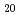 probed by muon spin relaxation
probed by muon spin relaxationHigemoto, Wataru; Sato, Kazuhiko*; Ito, Takashi; Oishi, Kazuki*; Saiga, Yuta*; Kosaka, Masashi*; Matsubayashi, Kazuyuki*; Uwatoko, Yoshiya*
Journal of Physics; Conference Series, 2462, p.012039_1 - 012039_5, 2023/03
Times Cited Count:0 Percentile:0.21(Physics, Applied)The cubic compound YbCo Zn
Zn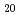 has huge electronic specific heat coefficient and its ground state could be located in the vicinity of the quantum critical point. Indeed, a magnetic long-range order was observed under pressure above 1-2 GPa. To investigate magnetic ground state, we have carried out muon spin relaxation measurements and confirm non-magnetic ground state with fluctuating tiny magnetic moment.
has huge electronic specific heat coefficient and its ground state could be located in the vicinity of the quantum critical point. Indeed, a magnetic long-range order was observed under pressure above 1-2 GPa. To investigate magnetic ground state, we have carried out muon spin relaxation measurements and confirm non-magnetic ground state with fluctuating tiny magnetic moment.
Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Nguyen, T. H.*; Le Ba, T.*; Tran, C. T.*; Nguyen, T. T.*; Doan, T. T. T.*; Do, V. K.; Watanabe, Masayuki; Pham, Q. M.*; Hoang, S. T.*; Nguyen, D. V.*; et al.
Hydrometallurgy, 213, p.105933_1 - 105933_11, 2022/08
Times Cited Count:9 Percentile:81.6(Metallurgy & Metallurgical Engineering)A continuous counter-current extraction for the selective recovery of thorium (Th) and uranium (U) from the Yen Phu (Vietnam) rare earth concentrate leach solutions was systematically studied. The primary amine N1923 was used as an extractant which was prepared in the isoparaffin IP-2028 diluent. Thorium and uranium were selectively recovered in a hydrometallurgical circuit established by continuous mixer-settler extraction, scrubbing, and back-extraction at the laboratory scale. The desired purity of Th and U can be achieved by managing the volume ratio of organic to aqueous phase (O/A ratio) in the corresponding steps. Highly pure Th and U were recovered from the pregnant back-extraction liquor and the raffinate, respectively, which have satisfactory properties for further processing of the subsequent nuclear materials.
Kaneko, Masashi
Nihon Genshiryoku Gakkai-Shi ATOMO , 64(1), p.30 - 34, 2022/01
, 64(1), p.30 - 34, 2022/01
Partitioning of minor actinides from rare earths is one of the most important techniques to develop group separation of high-level radioactive liquid waste. In this issue, the results of prediction of separation performance between minor actinides and rare earths observed in solvent extraction and the separation mechanism by means of using density functional theory are explained.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:3 Percentile:40.16(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:1 Percentile:13.58(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
Matsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Hydrometallurgy, 199, p.105539_1 - 105539_8, 2021/02
Times Cited Count:4 Percentile:32.7(Metallurgy & Metallurgical Engineering)The synergistic solvent extraction of lanthanide(III) with mixtures of di-(2-ethylhexyl)phosphoric acid (D2EHPA, A) and monoisodecyl phosphoric acid (MIDPA, B) in phosphonium-based ionic liquid was investigated. In the case of D2EHPA or MIDPA single extractant system, Ln(III) (Ln = Pr and Nd) was extracted as [LnA HA] or [LnB
HA] or [LnB HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (
HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (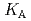 ,
, 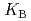 and
and 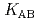 ) and the formation constants (
) and the formation constants (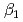 ,
, 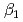 and
and 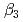 ), it was found that the extracted complex [TbHA
), it was found that the extracted complex [TbHA B
B or [DyHA
or [DyHA B
B ] was more stable than [LnA
] was more stable than [LnA HA] or [LnB
HA] or [LnB HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Sasaki, Yuji; Ono, Ryoma*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.597 - 607, 2021/01
Times Cited Count:2 Percentile:23.63(Chemistry, Analytical)To achieve trichotomic separation of light lanthanides (Ln), heavy Ln, and Am, batchwise multi-stage extractions using tetraoctyl-diglycolamide (TODGA) extractant from organic acids are studied. Malonic acid (MA) has high solubility in water and is used as the main component of the aqueous phase. It is clear that the separation factor (SF) for Nd/Am from MA and that for La/Am from MA + HNO are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO
are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO (1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
(1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
 -diketone extractant and a neutral ligand
-diketone extractant and a neutral ligandMatsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Solvent Extraction and Ion Exchange, 39(7), p.764 - 784, 2021/00
Times Cited Count:4 Percentile:22.03(Chemistry, Multidisciplinary)We investigated the solvent extraction of four rare earth (RE) elements (Pr, Nd, Tb, and Dy) from Nd-Fe-B magnets using mixtures of 1-(2-thienyl)-4,4,4,-trifluoro-1,3-butanedione (Htta) or 4,4,4-trifluoro-1-phenyl-1,3-butanedione (Hbfa) chelating extractants and tri-n-octylphosphine oxide (TOPO) neutral ligand in phosphonium based ionic liquids. A synergistic effect was observed for the extraction of the RE elements with the combination of extractant and neutral ligand. The separation of Tb(III) and Dy(III) from other RE(III) components was performed with seven extraction cycles.
 -tetraoctyl-diglycolamide from organic acid
-tetraoctyl-diglycolamide from organic acidSasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 49(10), p.1216 - 1219, 2020/10
Times Cited Count:9 Percentile:44.36(Chemistry, Multidisciplinary)Lanthanide (Ln) extractions from organic acids to  -dodecane by
-dodecane by  -tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio (
-tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio ( ) greater 1 was obtained for heavy Ln. Ln patterns (
) greater 1 was obtained for heavy Ln. Ln patterns ( (Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high
(Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high  values, the addition of HNO
values, the addition of HNO in aqueous phase is found to be effective.
in aqueous phase is found to be effective.
 -isopropylacrylamide) with diglycolamide-typed ligands
-isopropylacrylamide) with diglycolamide-typed ligandsSaga, Kaname*; Suzuki, Hideya; Matsumura, Tatsuro; Tsukahara, Takehiko*
Analytical Sciences, 35(4), p.461 - 464, 2019/04
Times Cited Count:3 Percentile:12.17(Chemistry, Analytical)The phase transition-based gelification phenomenon of poly- -isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
-isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
Tsukahara, Takehiko*; Suzuki, Hideya*; Matsumura, Tatsuro; Saga, Kaname*
Bunri Gijutsu, 49(4), p.221 - 225, 2019/04
no abstracts in English
Yamaguchi, Akiko*; Honda, Tasuku*; Tanaka, Masato*; Tanaka, Kazuya; Takahashi, Yoshio*
Geochemical Journal, 52(5), p.415 - 425, 2018/00
Times Cited Count:18 Percentile:67.29(Geochemistry & Geophysics)Ion-adsorption type REE deposits in weathered granite are main sources of REE essential for high-technology industries. However, these type deposits have not been searched in Japan. In this study, Bulk REE abundances (= REE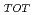 ), ion-exchangeable REE (= REE
), ion-exchangeable REE (= REE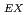 ) by ammonium chloride solution, and percentage of REE
) by ammonium chloride solution, and percentage of REE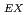 relative to REE
relative to REE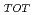 (= REE
(= REE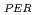 ) in fresh and weathered granite samples in Southwest Japan (i.e., Hiroshima and Shimane Prefectures) were determined. The REE
) in fresh and weathered granite samples in Southwest Japan (i.e., Hiroshima and Shimane Prefectures) were determined. The REE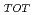 and REE
and REE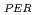 were comparable to those of ion-adsorption type REE deposits in China. Extended X-ray adsorption fine structure (EXAFS) spectra of REE in original samples and samples after the extraction of ion-exchangeable REE showed that (i) REE in samples with high REE
were comparable to those of ion-adsorption type REE deposits in China. Extended X-ray adsorption fine structure (EXAFS) spectra of REE in original samples and samples after the extraction of ion-exchangeable REE showed that (i) REE in samples with high REE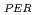 mainly forms outer-sphere complexes and (ii) the remaining REE in the rocks after the extraction forms inter-sphere complexes.
mainly forms outer-sphere complexes and (ii) the remaining REE in the rocks after the extraction forms inter-sphere complexes.
Iwatsuki, Teruki; Munemoto, Takashi*; Kubota, Mitsuru*; Hayashida, Kazuki; Kato, Toshihiro*
Applied Geochemistry, 82, p.134 - 145, 2017/05
Times Cited Count:9 Percentile:37.38(Geochemistry & Geophysics)This study investigated the behavior of rare earth elements (REEs) associated with suspended particles in deep granitic groundwater and in a sealed drift at a depth of 500 m in the Mizunami Underground Research Laboratory (URL) in Japan. Approximately 10% 60% of REEs in groundwater are associated with suspended particles. Carbonate particles in groundwater are most likely derived from in situ precipitation of supersaturated carbonate minerals such as calcite. Thermodynamic calculations show that the dissolved REE carbonate complexes in the closed drift decreased in the drift closure period. These complexes may have been absorbed or co-precipitated within the shotcrete on the drift wall. The usage of cement based materials would generate environmental conditions in which REEs are fundamentally immobile in and around the underground facilities.
60% of REEs in groundwater are associated with suspended particles. Carbonate particles in groundwater are most likely derived from in situ precipitation of supersaturated carbonate minerals such as calcite. Thermodynamic calculations show that the dissolved REE carbonate complexes in the closed drift decreased in the drift closure period. These complexes may have been absorbed or co-precipitated within the shotcrete on the drift wall. The usage of cement based materials would generate environmental conditions in which REEs are fundamentally immobile in and around the underground facilities.
Kirishima, Akira*; Kuno, Atsushi*; Amamiya, Hiroki; Kubota, Takumi*; Kimuro, Shingo*; Amano, Yuki; Miyakawa, Kazuya; Iwatsuki, Teruki; Mizuno, Takashi; Sasaki, Takayuki*; et al.
Chemosphere, 168, p.798 - 806, 2017/02
Times Cited Count:3 Percentile:10.09(Environmental Sciences)For better understanding of the migration behavior of minor actinides (MA) in deep groundwater, the interaction of doped rare earth elements (REEs) and components in Horonobe deep groundwater was studied. Appx. 10 ppb of rare earth elements, i.e., Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Tm and Yb were doped to the sample groundwater collected from a packed sections in borehole drilled from 140 m depth experiment drift of Horonobe underground research laboratory (URL), Hokkaido, Japan. Then, that groundwater was sequentially filtrated by 0.2 micron pore filter, 10 kDa, 3 kDa and 1 kDa of nominal molecular weight limit (NMWL) ultrafilters by keeping inert condition. After that, the filtrate solutions were analyzed by ICP-MS to determine the concentrations of retained REEs at each filtration steps, while the used filters were analyzed by the neutron activation analysis (NAA) and TOF-SIMS element mapping to know the amount and chemical speciation of trapped fraction of the REEs on each filter. A remarkable relation between the retention ratios of REEs in the filtrate solutions and the ionic radius was observed, i.e., smaller rare earth element solves more in liquid phase under the Horonobe groundwater condition. NAA and TOF-SIMS analyses revealed that certain portions of REEs were trapped by 0.2 micron pore filters as rare earth phosphates which corresponded with the predicted predominant species by a chemical equilibrium calculation for the Horonobe groundwater condition, while small portions of colloidal REEs were trapped by 10 kDa and 3 kDa NMWL ultrafilters. The result suggested that phosphate anion plays an important role in the chemical behavior of REEs in saline (seawater based) groundwater, which could be referred for the prediction of migration behavior of trivalent actinide released from the repository of radioactive waste in far future.
Murakami, Takuma; Sasamoto, Hiroshi; Mizuno, Takashi
Chikyu Kagaku, 50(4), p.299 - 317, 2016/12
Development of techniques for investigating the long-term migration of elements in deep underground is important with respect to safety assessment for the geological disposal of high-level radioactive waste. As one study of the migration of elements in deep underground, the distribution of REE, Th and U in sedimentary rock of Horonobe area in Hokkaido was investigated, and discussed whether the difference of hydrogeological structure and lithofacies influences on the distributions. As the results, it was considered that REE and Th were mainly retained in minerals originating from terrigenous clastic and secondary mineral occurred in early diagenesis, and their distributions were relatively homogenous in both the Koetoi and Wakkanai formations. Uranium was indicated to be maintained in the sedimentary rock until now after depositing in deep-sea sediment. It was considered that the U deposition was occurred by adsorption on organic matter and reductive precipitation with decomposition of organic matter. In addition, it was confirmed that the distributions of these elements were not influenced by the difference of hydrogeological and lithofacies.
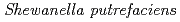
Fujimoto, Jun*; Tanaka, Kazuya; Watanabe, Naoko*; Takahashi, Yoshio*
Hydrometallurgy, 166, p.80 - 86, 2016/12
Times Cited Count:6 Percentile:31.22(Metallurgy & Metallurgical Engineering)We examined recovery of REEs in Fe-Mn nodules by using 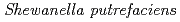 (Fe-reducing bacterium). In this method, Fe-Mn nodule decomposition and REE recovery were achieved simultaneously in a single solution system. Fe-Mn nodules were reductively decomposed in NaCl solution under anaerobic conditions with daily addition of sodium lactate as an electron donor. During the decomposition of Fe-Mn nodule, REEs released from the Fe-Mn nodule were adsorbed on bacterial cells. Of the conditions studied here, the best REE adsorption rates were obtained with 0.5M NaCl solution at pH7 with daily addition of 1 mmol sodium lactate.
(Fe-reducing bacterium). In this method, Fe-Mn nodule decomposition and REE recovery were achieved simultaneously in a single solution system. Fe-Mn nodules were reductively decomposed in NaCl solution under anaerobic conditions with daily addition of sodium lactate as an electron donor. During the decomposition of Fe-Mn nodule, REEs released from the Fe-Mn nodule were adsorbed on bacterial cells. Of the conditions studied here, the best REE adsorption rates were obtained with 0.5M NaCl solution at pH7 with daily addition of 1 mmol sodium lactate.
Munemoto, Takashi; Omori, Kazuaki*; Iwatsuki, Teruki
Chemical Geology, 417, p.58 - 67, 2015/12
Times Cited Count:32 Percentile:73.58(Geochemistry & Geophysics)Rare earth elements (REEs) combined with yttrium (YREE) in deep groundwater from granite and fracture-filling calcite are being studied at the Mizunami Underground Research Laboratory (MIU, Tono area, central Japan).